X-ray Emission from Atoms
Inside the Atom
An atom has a nucleus, which contains particles of positive charge
(protons) and particles of
neutral charge (neutrons).
Surrounding the nucleus of an atom are shells of electrons - small negatively charged
particles. Each shell has a specific energy associated with it.
Within these shells the electrons move around the nucleus of the atom.
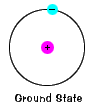 |
The ground state of an electron, the energy level it
normally occupies, is the state of lowest energy for that
electron. (Note that electron doesn't actually move about the nucleus
in a circle. See The Quantum Story below.) |
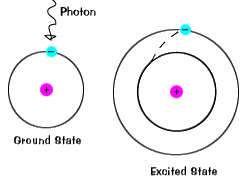
| When an electron occupies an energy shell greater than its ground
state, it is in an excited state. An electron can become excited
if it is given extra energy, such as when it absorbs a
photon or if collides with a
nearby atom or particle. |
 |
 |
An electron does not stay in an excited state for very long - it soon
returns to the ground states. When it does so, a photon is emitted
that has the same energy as the difference in the energy level
between the excited state and the ground state.
|
So an electron moving from one energy shell to a lower one emits a
photon of a specific energy. Since the energy and wavelength of the
photon are related, we see this photon at a specfic wavelength in the
spectrum. We refer to these as a "line" in the spectrum. Because
there are many energy shells in any particular atom, there are many
different possible energies with different initial and final values.
When an atom is in an excited state, the electron can drop all the
way to the ground state, or stop in an intermediate level.
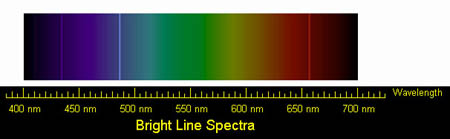
Below is the optical spectrum for
hydrogen.
The distinct lines near 435 nm, 487 nm, and 655 nm show transitions
from the 5th, 4th and 3rd energy shells, respectively, into the 2nd
energy shell.
Click on image for larger version.
A hydrogen atom has one proton and one electron. That makes it
easy to understand, and scientists can calculate exactly what energy
the electron has in each shell. But hydrogen is also the least energetic
element. Even the most energetic line hydrogen emits (when an
electron drops down from the second shell to the first) has only
enough energy to be an ultraviolet photon. So hydrogen atoms do
not emit X-rays!
So what elements emit X-ray lines? The more protons an element has,
more energetic its lines can be. Carbon atoms (6 protons each) can
emit X-rays. But carbon lines are at the low end of X-rays. Many
X-ray instruments cannot detect these photons. Of the common elements
in the universe, iron (26 protons) and oxgen (8 protons) usually are
the two most prominent sources of X-ray lines.
When first learning about the structure of atoms, it is common to hear
about the electrons being in "orbitals" that have different energies.
We then picture the electron being in a specific location in that orbit.
This is convenient, but not the most accurate way to
model the electrons in an atom.
Although each shell does have a precise energy, the position of the
electron is envisioned as being smeared out into an "electron cloud"
surrounding the nucleus. So, in our quantum story, we don't know the
precise location of the electron, only where it is likely to be.
So instead of electrons moving about the nucleus in a plane (like the
planets in the solar system) electrons move about the nucleus in
three-dimensions. Further, the electron cloud of each
energy level of an atom has a different shape. There are mathematical
equations which will tell you the probability of the electron's location
within that shell.
Let's consider the hydrogen atom.

Probable locations of the electron in the
ground state of the Hydrogen atom.
|
At the left is a graph of the probable location of the electron in the
ground state. The nucleus is at the center, and where the graph is
brightest is where the electron is most likely to lie. What you see
here is a cross section. That is, you have to imagine the picture
rotated around the vertical axis. So the region inhabited by
this electron looks like a disk in this graph, but it is actually
a sphere. |
|
To the right is an excited state of hydrogen.
Notice that at the center, where the nucleus is, the picture
is dark, indicating that the electron is unlikely to be there. The two bright
regions, where the electron is most likely to
be found, are really just one region. Remember, you have to
mentally rotate this around a vertical axis, so that in three dimensions
the light region is really doughnut shaped.
|

Probable locations of the electron in an
excited state of Hydrogen.
|
|
